The acid dissociation constant pKa is obtained by neutralization titration.
If “PK1” is entered in the formula, the estimated value of pKa can be calculated.
If a polyvalent acid is being measured, and multiple endpoints are obtained, then enter “PK2” and “PK3” in the formula to calculate the respective second and third stage dissociation constants. In this case, the results are conditional on clear separation of the endpoints. Note that pKa measurements are limited to aqueous solutions, and the pH must be plotted on the vertical axis of the titration curve. The calculation of pKa with KEM potentiometric titrators is based on the principles noted below.
The acid dissociation constant is defined as follows.
FAQAutomatic Potentiometric Titrators

Automatic Potentiometric Titrators
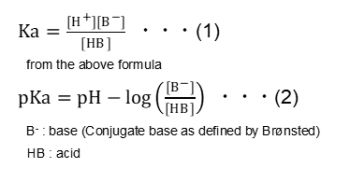
From formula (2) noted above, [B-] = [HB], or in other words, the pH when half-neutralized indicates the pKa value.
The assumption that [B-] = [HB] at the half-equivalence point is an approximation, but the error is not large for pKa values of 3 to 11. For this reason, with KEM potentiometric titrators, the pH at 1/2 the titration quantity required up to the equivalence point is taken as pKa.